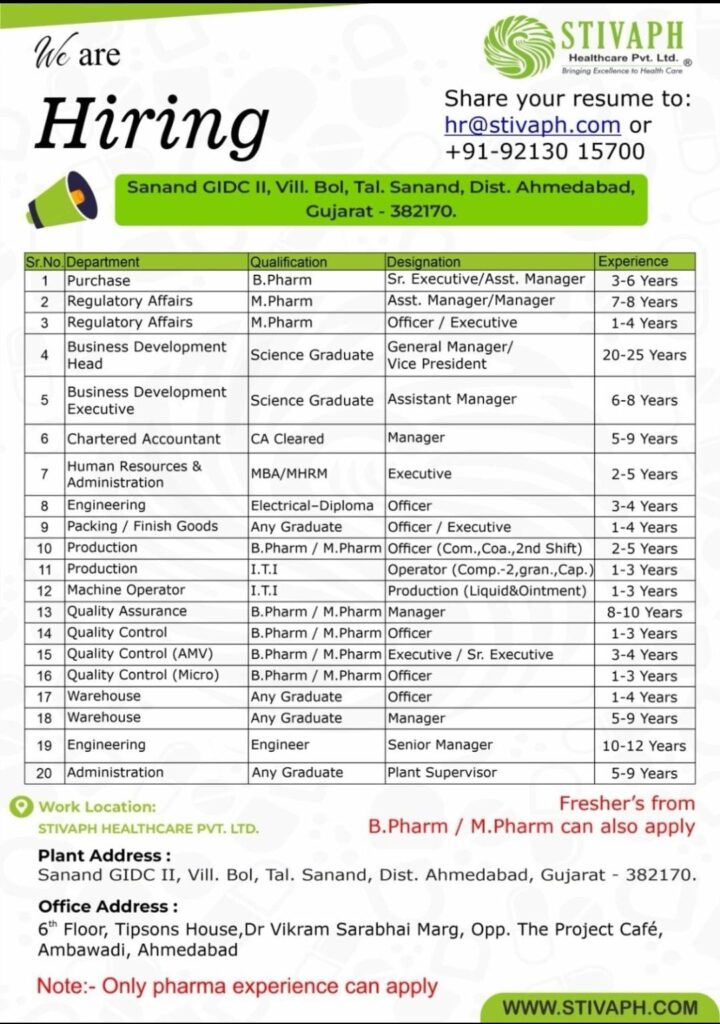
Production QA QC Regulatory Affairs HR Jobs in Gujrat
Stivaph Healthcare Pvt. Ltd. is a rapidly growing pharmaceutical company committed to quality, compliance, and innovation. The company operates a modern manufacturing facility at Sanand GIDC II and offers a professional work environment with career growth opportunities across technical, regulatory, production, and corporate functions.
Job Location
- Plant Location: Sanand GIDC II, Vill. Bol, Tal. Sanand, Dist. Ahmedabad, Gujarat – 382170
- Office Location: 6th Floor, Tipsons House, Dr. Vikram Sarabhai Marg, Ambawadi, Ahmedabad
Production QA QC Regulatory Affairs HR Jobs in Gujrat at Stivaph Healthcare
Below is the detailed list of available positions:
Purchase Department
- Qualification: B.Pharm
- Designation: Sr. Executive / Assistant Manager
- Experience: 3–6 Years
Regulatory Affairs
- Qualification: M.Pharm
- Designation: Assistant Manager / Manager
- Experience: 7–8 Years
- Qualification: M.Pharm
- Designation: Officer / Executive
- Experience: 1–4 Years
Business Development
- Qualification: Science Graduate
- Designation: General Manager / Vice President
- Experience: 20–25 Years
- Qualification: Science Graduate
- Designation: Assistant Manager
- Experience: 6–8 Years
Finance & HR
- Chartered Accountant:
- Qualification: CA Cleared
- Designation: Manager
- Experience: 5–9 Years
- HR & Administration:
- Qualification: MBA / MHRM
- Designation: Executive
- Experience: 2–5 Years
Engineering
- Qualification: Electrical Diploma
- Designation: Officer
- Experience: 3–4 Years
- Qualification: Engineer
- Designation: Senior Manager
- Experience: 10–12 Years
Production Department
- Qualification: B.Pharm / M.Pharm
- Designation: Officer (Compression, Coating, 2nd Shift)
- Experience: 2–5 Years
- Qualification: ITI
- Designation: Operator (Compression, Granulation, Capsule)
- Experience: 1–3 Years
- Machine Operator (Liquid & Ointment):
- Qualification: ITI
- Experience: 1–3 Years
Quality Department
- Quality Assurance:
- Qualification: B.Pharm / M.Pharm
- Designation: Manager
- Experience: 8–10 Years
- Quality Control:
- Qualification: B.Pharm / M.Pharm
- Designation: Officer
- Experience: 1–3 Years
- QC (AMV):
- Qualification: B.Pharm / M.Pharm
- Designation: Executive / Sr. Executive
- Experience: 3–4 Years
- QC Microbiology:
- Qualification: B.Pharm / M.Pharm
- Designation: Officer
- Experience: 1–3 Years
Warehouse
- Qualification: Any Graduate
- Designation: Officer
- Experience: 1–4 Years
- Qualification: Any Graduate
- Designation: Manager
- Experience: 5–9 Years
Administration
- Qualification: Any Graduate
- Designation: Plant Supervisor
- Experience: 5–9 Years
Production QA QC Regulatory Affairs HR Jobs in Gujrat Eligibility Criteria
- Candidates must have relevant pharma industry experience
- Freshers from B.Pharm / M.Pharm can also apply (for selected roles)
- Only candidates with pharmaceutical background are eligible
How to Apply for Production QA QC Regulatory Affairs HR Jobs in Gujrat
Interested and eligible candidates can share their updated resume through:
- Email: hr@stivaph.com
- Contact Number: +91-92130-15700
Mention the applied department and designation clearly in the subject line.