Mepro Pharmaceuticals Job Vacancy 2025
Mepro Pharmaceuticals Pvt. Ltd. (Unit-III), committed to humanity, is on a hiring spree! The company is looking to expand its team in Vadodara, Gujarat, and has announced multiple job openings across various departments. If you are an experienced professional in the pharmaceutical sector looking for your next career move, this could be the perfect opportunity for you.
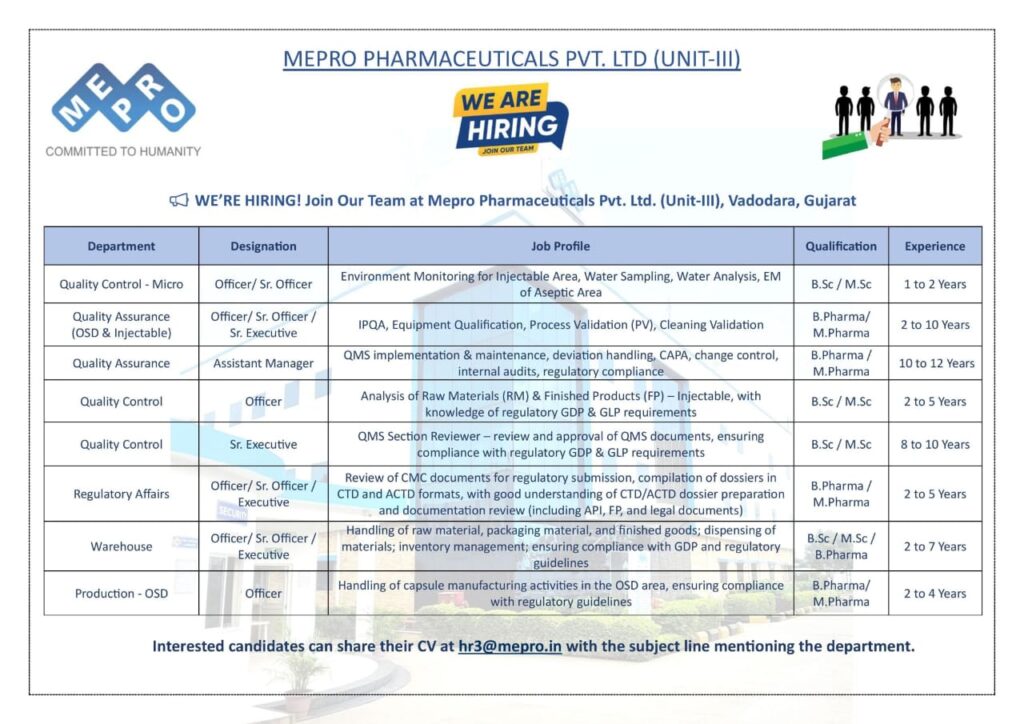
Here is a detailed breakdown of the available job vacancies.
Available Positions and Eligibility Criteria Mepro Pharmaceuticals Job Vacancy 2025
Mepro Pharmaceuticals is seeking skilled and dedicated individuals for the following departments:
Quality Control
- Designation: Officer/Sr. Officer (Micro) & Officer
- Job Profile:
- For the Officer/Sr. Officer role, the responsibilities include environmental monitoring for injectable areas, water sampling, water analysis, and EM of the aseptic area.
- The Officer position involves the analysis of raw materials and finished products, specifically injectables, with a strong understanding of regulatory GDP & GLP requirements.
- Qualification: B.Sc/M.Sc
- Experience: 1 to 2 years for the micro role and 2 to 5 years for the general officer position.
- Designation: Sr. Executive
- Job Profile: QMS Section review, approval of QMS documents, and ensuring compliance with regulatory GDP & GLP requirements.
- Qualification: B.Sc/M.Sc
- Experience: 8 to 10 years.
Quality Assurance (OSD & Injectable)
- Designation: Officer/Sr. Officer/Sr. Executive
- Job Profile: The role focuses on IPOA, equipment qualification, process validation (PV), and cleaning validation.
- Qualification: B.Pharma/M.Pharma
- Experience: 2 to 10 years.
Quality Assurance
- Designation: Assistant Manager
- Job Profile: This role involves QMS implementation & maintenance, deviation handling, CAPA, change control, internal audits, and regulatory compliance.
- Qualification: B.Pharma/M.Pharma
- Experience: 10 to 12 years.
Regulatory Affairs
- Designation: Officer/Sr. Officer/Executive
- Job Profile: Responsibilities include the review of CMC documents for regulatory submission, compilation of dossiers in CTD and ACTD formats, and document review.
- Qualification: B.Pharma/M.Pharma
- Experience: 2 to 5 years.
Warehouse
- Designation: Officer/Sr. Officer/Executive
- Job Profile: Handling of raw material, packaging material, and finished goods, inventory management, and ensuring compliance with GDP and regulatory guidelines.
- Qualification: B.Sc/M.Sc/B.Pharma
- Experience: 2 to 7 years.
Production – OSD
- Designation: Officer
- Job Profile: Handling of capsule manufacturing activities in the OSD area while ensuring compliance with regulatory guidelines.
- Qualification: B.Pharma/M.Pharma
- Experience: 2 to 4 years.
How to Apply Mepro Pharmaceuticals Job Vacancy 2025
Interested and eligible candidates are requested to send their updated CV to hr3@mepro.in