BDR Pharma Vacanccy Regulatory Affairs 2025
Are you a meticulous professional with a passion for ensuring pharmaceutical products meet global standards? BDR Pharmaceuticals Internationals Pvt. Ltd. is actively seeking talented individuals to join our dynamic Regulatory Affairs Department. This is your chance to contribute to a leading pharmaceutical company and make a real impact on patient health worldwide.
At BDR Pharmaceuticals, we believe in fostering a collaborative environment where innovation and expertise thrive. Our Regulatory Affairs team is at the forefront of ensuring compliance, navigating complex international guidelines, and bringing life-changing medications to market. If you’re ready for a challenging yet rewarding career, explore our current openings BDR Pharma Vacanccy Regulatory Affairs 2025!
Current BDR Pharma Vacanccy Regulatory Affairs 2025 :
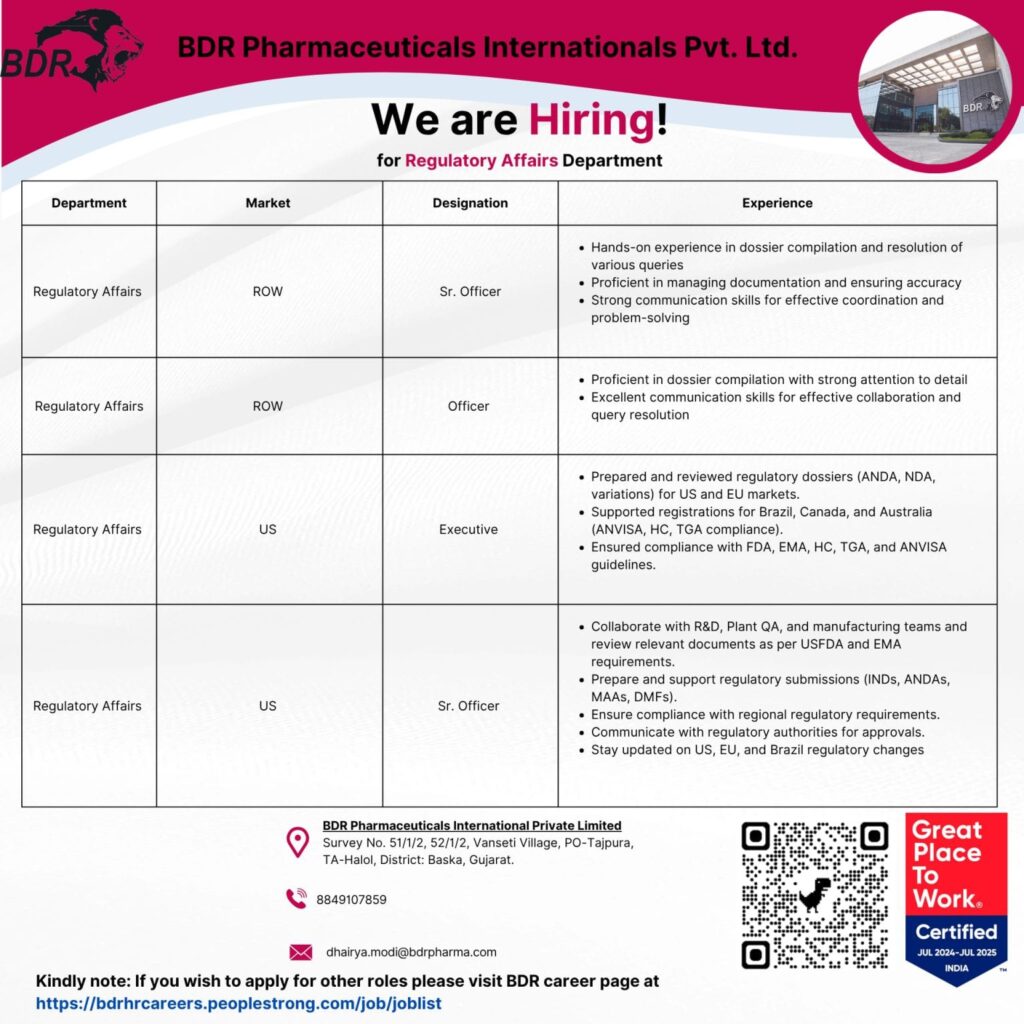
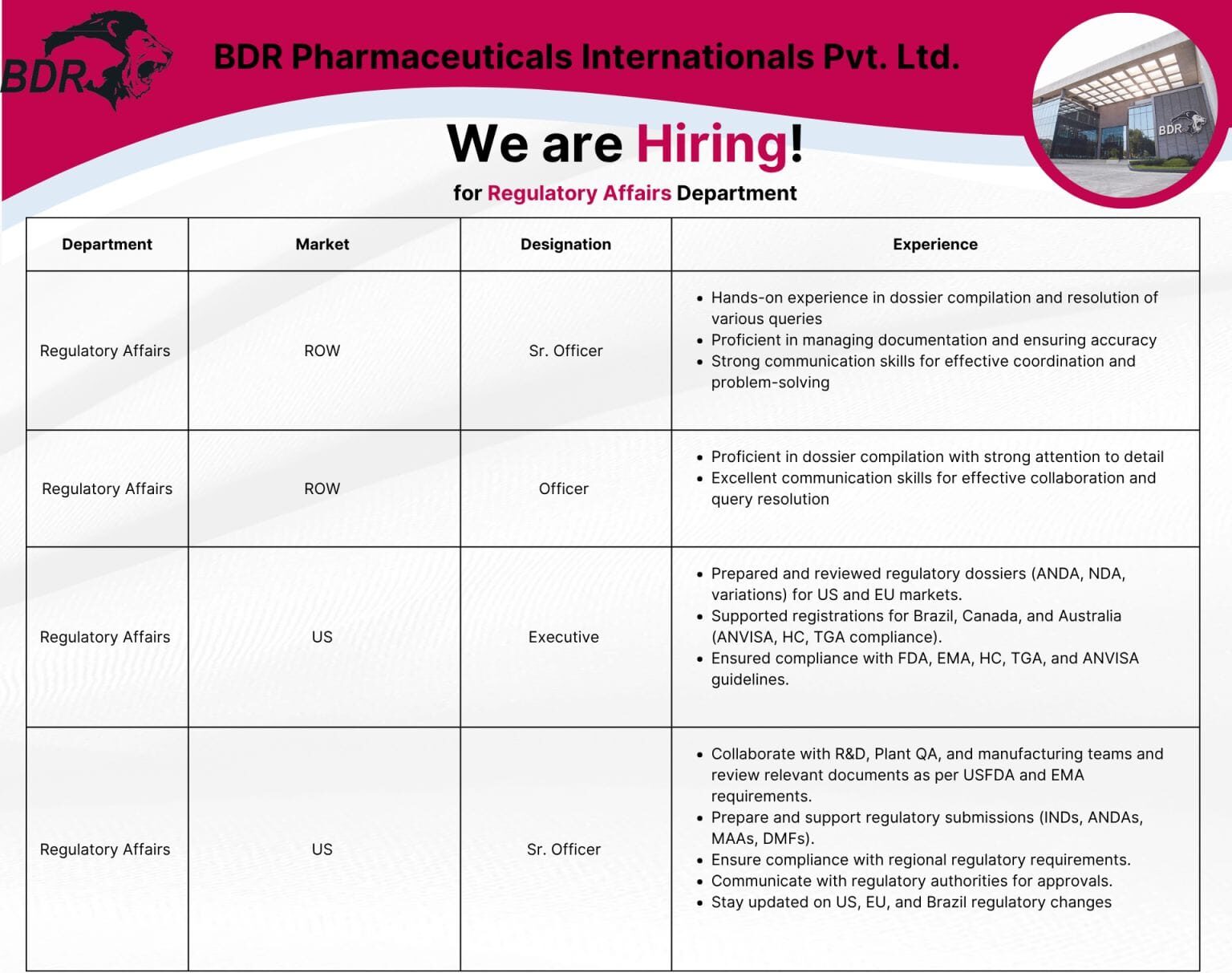
We have exciting opportunities across various experience levels and markets, including India (ROW) and the US. Whether you’re an experienced professional or looking to take the next step in your Regulatory Affairs career, we have a role for you.
BDR Pharma Vacanccy Regulatory Affairs 2025
- Sr. Officer – Regulatory Affairs (ROW Market): This role demands hands-on experience in dossier compilation, query resolution, and strong communication skills. You’ll be proficient in managing documentation and ensuring accuracy for our ROW market operations.
- Officer – Regulatory Affairs (ROW Market): Focus on detailed dossier compilation, excellent communication, and effective collaboration to support our ROW market initiatives.
- Executive – Regulatory Affairs (US Market): Prepare and review regulatory dossiers (ANDA, NDA, variations) for US and EU markets. This role involves submitting applications to USFDA, Health Canada, and ANVISA, ensuring compliance with global guidelines.
- Sr. Officer – Regulatory Affairs (US Market): Collaborate with R&D, Plant QA, and manufacturing teams to review relevant documents as per USFDA and EMA requirements. Prepare and support regulatory submissions (INDs, ANDAs, MAAs, DMFs) and ensure compliance with regional regulatory requirements. Stay updated on US, EU, and Brazil regulatory changes.
Why Choose BDR Pharmaceuticals?
We are proud to be a “Great Place To Work – Certified July 2024-July 2025” company, reflecting our commitment to employee satisfaction and a supportive work culture. You’ll be part of a team that values precision, strategic thinking, and dedication to excellence.
How to Apply BDR Pharma Vacanccy Regulatory Affairs 2025:
If you wish to apply for other roles or learn more about BDR career opportunities, please visit our career page at: https://bdrhrcareers.peoplestrong.com/job/joblist
Alternatively, you can send your resume to dhairya.modi@bdrpharma.com or call us at 8849107859 for more information.
Location: Survey No. 51/1/2, 52/1/2, Vanseti Village, PO-Tajpura, TA-Halol, District: Baska, Gujarat.
Don’t miss this opportunity to advance your career in Regulatory Affairs with a company that’s making a difference!