Umedica Laboratories Walk-In Interview August 2025
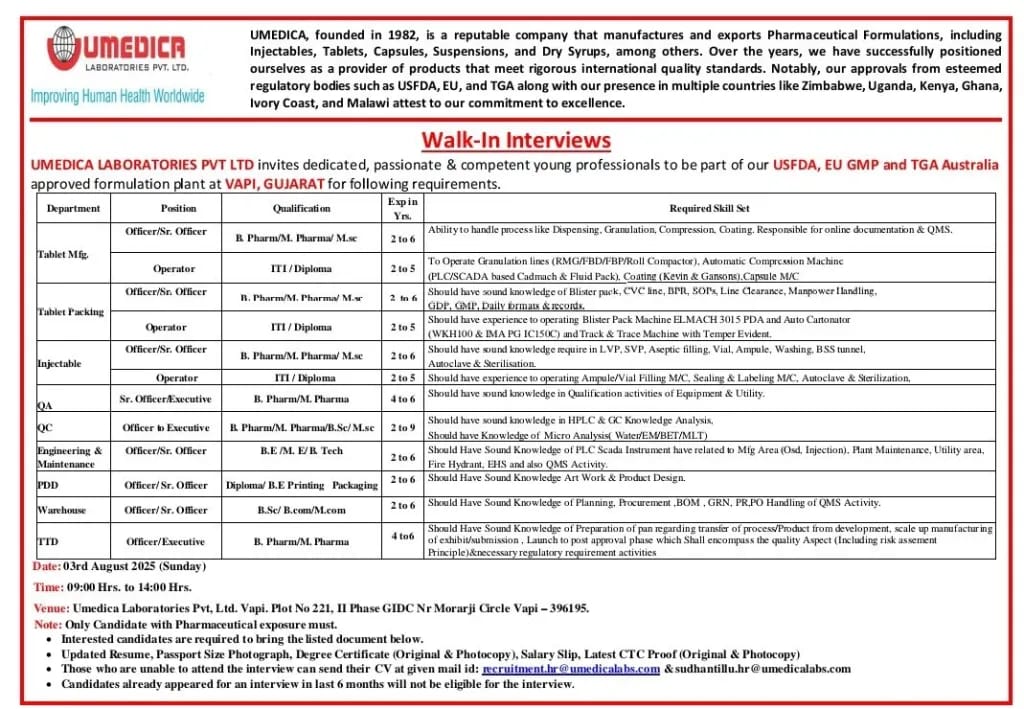
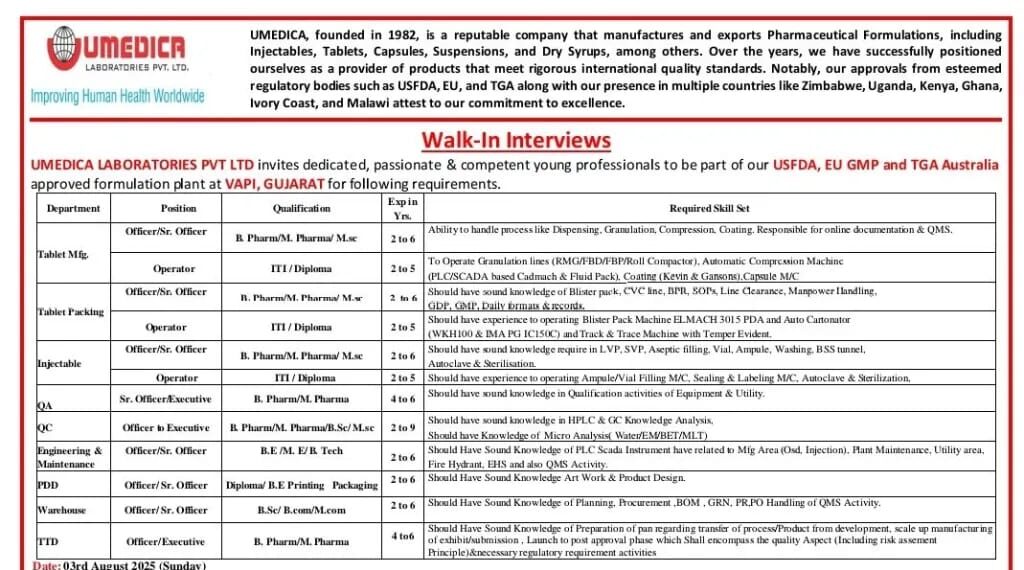
Umedica Laboratories Pvt Ltd, a reputed pharmaceutical company established in 1982, is conducting a Walk-In Interview for multiple vacancies at its USFDA, EU GMP, and TGA Australia-approved formulation plant located in Vapi, Gujarat. The company manufactures and exports high-quality pharmaceutical formulations, including Injectables, Tablets, Capsules, Suspensions, and Dry Syrups, meeting international regulatory standards.
Interview Details
- Date: 3rd August 2025 (Sunday)
- Time: 09:00 AM to 02:00 PM
- Venue:
Umedica Laboratories Pvt. Ltd.,
Plot No 221, Phase GIDC, Near Morarji Circle, Vapi – 396195, Gujarat
Eligibility & Requirements
- Only candidates with pharmaceutical experience are eligible.
- Bring updated resume, passport size photograph, degree certificates, salary slip, and latest CTC proof (original & photocopy).
- Candidates who have appeared for an interview in the last 6 months are not eligible.
Available Positions
Tablet Manufacturing
- Officer/Sr. Officer – B.Pharm/M.Pharm/M.Sc | 2–6 years | Expertise in dispensing, granulation, compression, coating, and QMS.
- Operator – ITI/Diploma | 2–5 years | Operation of RMG/FBD/FBP/Roll Compactor, capsule M/C, coating, and compression machines.
Tablet Packing
- Officer/Sr. Officer – B.Pharm/M.Pharm/M.Sc | 2–6 years | Knowledge of blister pack, CVC line, BFL, SOHA, line clearance, and manpower handling.
- Operator – ITI/Diploma | 2–5 years | Experience in Blister Pack Machine (ElMACH 3015 PDA) and auto cartoner machines.
Injectable
- Officer/Sr. Officer – B.Pharm/M.Pharm/M.Sc | 2–6 years | Experience in vial, ampoule filling, sealing, labeling, and sterilization.
- Operator – ITI/Diploma | 2–5 years | Knowledge of LVP, SVP, aseptic filling, and utilities.
Quality Assurance (QA)
- Sr. Officer/Executive – B.Pharm/M.Pharm | 4–6 years | Experience in qualification and equipment utility audits.
Quality Control (QC)
- Officer to Executive – B.Pharm/M.Pharm/M.Sc/M.Sc (Micro) | 2–5 years | Proficiency in HPLC & GC, Micro analysis, water/EM/BET/MLT.
Engineering & Maintenance
- Officer/Sr. Officer – B.E/M.E/B.Tech | 2–5 years | Knowledge of PLC/SCADA, utilities, HVAC, and MEP.
Product Development Department (PDD)
- Officer/Sr. Officer – Diploma/B.E in Printing & Packaging | 2–5 years | Expertise in packaging design, BOM, GRN, and PR/PO handling.
Warehouse
- Officer/Sr. Officer – B.Sc/B.Com/B.Pharm | 2–6 years | Skills in planning, inventory, and documentation (BOM, GRN, QMS).
Technology Transfer Department (TTD)
- Officer/Executive – B.Pharm/M.Pharm | 4–6 years | Process transfer from development to scale-up, risk assessment, and technical documentation.
Key Highlights of Umedica Laboratories
- Global presence in over 70 countries including Zimbabwe, Uganda, Kenya, Ghana, Ivory Coast, and Malawi.
- State-of-the-art manufacturing facilities compliant with USFDA, EU, and TGA standards.
- Opportunities to work on advanced formulations and international pharmaceutical projects.