Alembic Walk-In Drive 2026 in Vapi
Alembic Pharmaceuticals Limited, a Great Place to Work® certified company, is conducting a Walk-In Drive in Vapi for its Formulation and API manufacturing sites near Vadodara. This hiring drive is open for Production, Packing, and API professionals with relevant pharmaceutical experience.
This is a golden opportunity to join one of India’s most reputed pharma companies and build a long-term career.
Company Name
Alembic Pharmaceuticals Limited
Alembic Walk-In Drive 2026 in Vapi Details
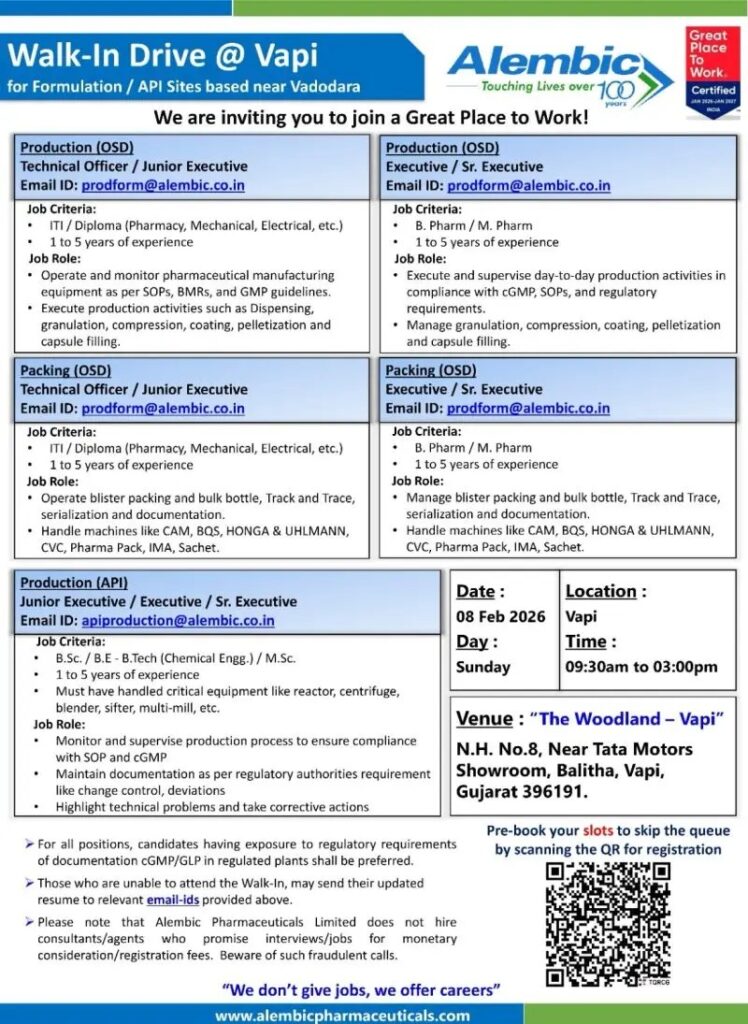
- Date: 08 February 2026
- Day: Sunday
- Time: 09:30 AM to 03:00 PM
- Location: Vapi
- Venue:
The Woodland – Vapi
N.H. No. 8, Near Tata Motors Showroom,
Balitha, Vapi, Gujarat – 396191
Job Openings at Alembic Pharmaceuticals
Production (OSD)
Technical Officer / Junior Executive
Qualification:
- ITI / Diploma (Pharmacy, Mechanical, Electrical, etc.)
Experience:
- 1 to 5 Years
Job Responsibilities:
- Operate and monitor pharmaceutical manufacturing equipment
- Execute production activities as per SOPs, BMRs, and cGMP
- Perform dispensing, granulation, compression, coating, pelletization, and capsule filling
Email: prodform@alembic.co.in
Executive / Senior Executive
Qualification:
- B.Pharm / M.Pharm
Experience:
- 1 to 5 Years
Job Responsibilities:
- Supervise day-to-day production operations
- Ensure compliance with cGMP, SOPs, and regulatory standards
- Manage OSD processes including granulation, compression, coating, pelletization, and capsule filling
Email: prodform@alembic.co.in
Packing (OSD)
Technical Officer / Junior Executive
Qualification:
- ITI / Diploma (Pharmacy, Mechanical, Electrical, etc.)
Experience:
- 1 to 5 Years
Job Responsibilities:
- Operate blister packing and bulk bottle lines
- Handle Track & Trace, serialization, and documentation
- Operate machines like IMA, CAM, BQS, HONGA, UHLMANN, CVC, Pharma Pack, IMA Sachet
Email: prodform@alembic.co.in
Executive / Senior Executive
Qualification:
- B.Pharm / M.Pharm
Experience:
- 1 to 5 Years
Job Responsibilities:
- Manage packing operations and compliance documentation
- Supervise blister and bottle packing lines
- Ensure smooth execution of serialization and regulatory requirements
Email: prodform@alembic.co.in
Production (API)
Junior Executive / Executive / Senior Executive
Qualification:
- B.Sc / B.E / B.Tech (Chemical Engineering) / M.Sc
Experience:
- 1 to 5 Years
Job Responsibilities:
- Monitor and supervise API production processes
- Operate critical equipment like reactors, centrifuges, blenders, sifters, multi-mill
- Maintain documentation as per cGMP and regulatory guidelines
- Handle deviations, change controls, and corrective actions
Email: apiproduction@alembic.co.in
Important Notes
- Candidates with GMP / GLP documentation experience in regulated plants will be preferred
- Candidates unable to attend the walk-in may email their updated resume
- Alembic Pharmaceuticals does not charge any fees for recruitment
- Beware of fraudulent job offers or agents
Why Join Alembic Pharmaceuticals?
- Great Place to Work® certified organization
- Strong regulatory exposure
- Stable career growth in formulation & API manufacturing
- Employee-centric work culture