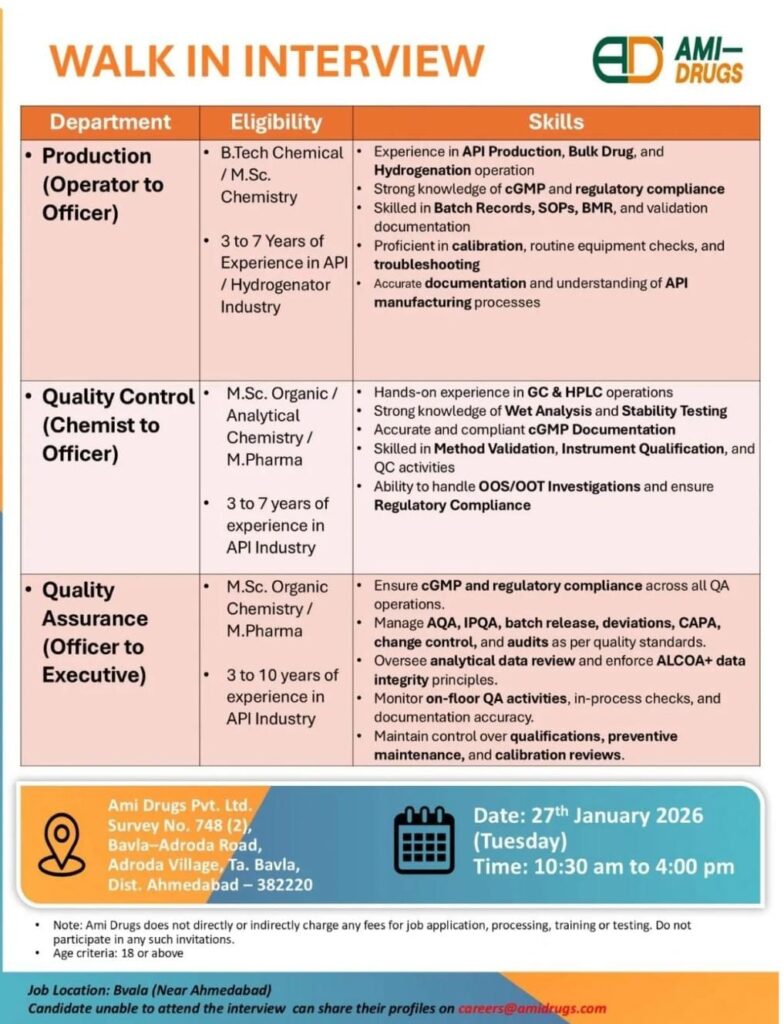
Ami Drugs Walk-In Interview 2026
If you are looking for API pharma jobs near Ahmedabad, this is a great opportunity. Ami Drugs Pvt. Ltd. is conducting a walk-in interview on 27th January 2026 for experienced professionals in Production, Quality Control (QC), and Quality Assurance (QA) departments.
This hiring drive is ideal for candidates with API industry experience who want to grow their careers with a reputed pharmaceutical company.
About Ami Drugs Pvt. Ltd.
Ami Drugs Pvt. Ltd. is a well-established API pharmaceutical manufacturing company known for its strong focus on cGMP compliance, quality systems, and regulatory standards. The company operates from Bavla–Adroda Road near Ahmedabad and offers stable long-term career growth in the API sector.
Walk-In Interview Details
- Date: 27th January 2026 (Tuesday)
- Time: 10:30 AM to 4:00 PM
- Job Location: Bavla (Near Ahmedabad), Gujarat
Interview Venue
Ami Drugs Pvt. Ltd.
Survey No. 748 (2),
Bavla–Adroda Road,
Adroda Village, Ta. Bavla,
Dist. Ahmedabad – 382220
Open Positions & Eligibility
1. Production Department (Operator to Officer)
Eligibility:
- B.Tech Chemical / M.Sc. Chemistry
- 3 to 7 years of experience in API / Hydrogenation industry
Key Skills Required:
- Experience in API Production, Bulk Drug & Hydrogenation operations
- Strong knowledge of cGMP & regulatory compliance
- Hands-on experience with Batch Records, SOPs, BMR & validation
- Calibration, routine equipment checks & troubleshooting
- Accurate documentation and understanding of API manufacturing processes
2. Quality Control (Chemist to Officer)
Eligibility:
- M.Sc. Organic Chemistry / Analytical Chemistry / M.Pharm
- 3 to 7 years of experience in API industry
Key Skills Required:
- Hands-on experience in GC & HPLC operations
- Strong knowledge of Wet Analysis & Stability Testing
- cGMP documentation & compliance
- Method Validation, Instrument Qualification & QC activities
- Handling OOS / OOT investigations and regulatory compliance
3. Quality Assurance (Officer to Executive)
Eligibility:
- M.Sc. Organic Chemistry / M.Pharm
- 3 to 10 years of experience in API industry
Key Skills Required:
- Ensure cGMP and regulatory compliance across QA operations
- Manage AQA, IPQA, batch release, deviations, CAPA & change control
- Handle audits as per quality standards
- Review analytical data and enforce ALCOA+ data integrity principles
- Monitor on-floor QA activities, in-process checks & documentation accuracy
- Control qualifications, preventive maintenance & calibration reviews
Unable to Attend the Walk-In?
Candidates who are unable to attend the interview can share their profiles via email:
📩 Email: careers@amidugs.com